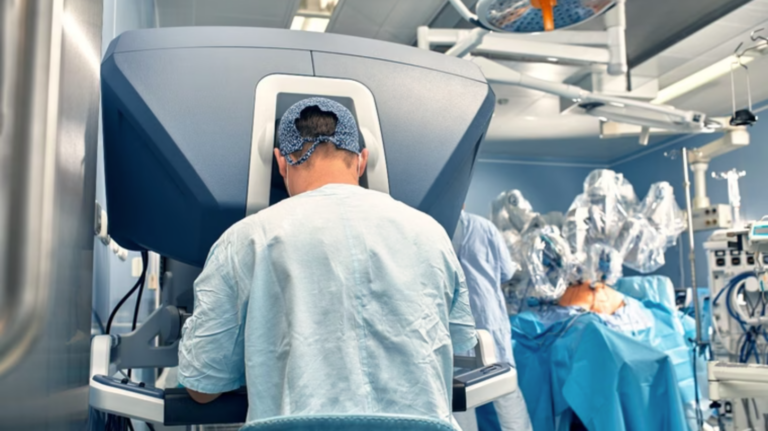
The UK’s National Institute for Health and Care Excellence (NICE) has granted conditional approval for 11 robotic surgery systems to be used across NHS centres, signalling a major step forward in the adoption of automation for soft tissue and orthopaedic procedures.
Five systems for soft tissue operations – such as tumour removal, gallbladder surgery and hernia repair – and six systems for orthopaedic procedures, including full and partial knee and hip replacements, have been approved under NICE’s Early Value Assessment programme.
The initiative allows these technologies to be used while data on their cost-effectiveness is gathered over a three-year period.
The systems, which range in cost from £500,000 to £1.5m, include console-controlled and handheld variants capable of highly precise movements surpassing those of the human hand.
Surgeons and patient groups have reported significant benefits, including reduced pain, faster recovery times, shorter hospital stays and less scarring.
According to NICE, the robotic systems are already showing a marked shift in uptake, with non-urological robotic surgeries rising from 20% in 2011/12 to 49% by 2023/24.
“Robot-assisted surgery may help overcome key limitations of conventional techniques through precise movements and enhanced 3D visualisation,” said Dr Anastasia Chalkidou, programme director of NICE’s HealthTech programme.
“Both applications could benefit patients who might not otherwise be candidates for minimally invasive approaches.”
Orthopaedic robot-assisted surgery is experiencing the fastest growth, increasing from around 300 cases in 2018/19 to over 4,000 in 2023/24, driven by expanding capabilities and growing clinician confidence.
Professor Sir Stephen Powis, NHS national medical director, added: “This is fantastic news for patients.
“Robot-assisted surgery is crucial to the future of high-quality healthcare and will be a vital element of the upcoming 10 Year Health Plan.”
John McGrath, chair of NHS England’s steering committee for robotic assisted surgery, confirmed national guidance on implementation will be published this month.
“We are working to ensure equitable access and develop NHS-wide surgical expertise so this technology can have the greatest possible impact,” he said.
Companies offering the systems must maintain regulatory compliance, including NHS England’s Digital Technology Assessment Criteria (DTAC), and commit to ongoing evidence generation.
NICE will reassess the guidance in three years to determine whether these robotic systems should become standard practice across the NHS.
Join more than 10,000 industry leaders at Robotics and Automation Exhibition on 18-19 March 2026, at the NEC Birmingham to explore cutting-edge technologies, connect with peers and discover the latest innovations shaping the future of manufacturing, engineering and logistics. Register your interest now to secure your place at this premier event!